
| Stent Diameter (mm) | 8 mm | 13 mm | 16 mm | 20 mm | 24 mm | 28 mm | 32 mm | 36 mm | 40 mm | 43 mm | 47 mm | Over Expansion (mm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | TF20008 | TF20013 | TF20016 | TF20020 | TF20024 | TF20028 | TF20032 | TF20036 | TF20040 | TF20043 | TF20047 | 3.5 |
| 2.25 | TF22508 | TF22513 | TF22516 | TF22520 | TF22524 | TF22528 | TF22532 | TF22536 | TF22540 | TF22543 | TF22547 | 3.5 |
| 2.5 | TF25008 | TF25013 | TF25016 | TF25020 | TF25024 | TF25028 | TF25032 | TF25036 | TF25040 | TF25043 | TF25047 | 3.5 |
| 2.75 | TF27508 | TF27513 | TF27516 | TF27520 | TF27524 | TF27528 | TF27532 | TF27536 | TF27540 | TF27543 | TF27547 | 4.5 |
| 3 | TF30008 | TF30013 | TF30016 | TF30020 | TF30024 | TF30028 | TF30032 | TF30036 | TF30040 | TF30043 | TF30047 | 4.5 |
| 3.5 | TF35008 | TF35013 | TF35016 | TF35020 | TF35024 | TF35028 | TF35032 | TF35036 | TF35040 | TF35043 | TF35047 | 4.5 |
| 4 | TF40008 | TF40013 | TF40016 | TF40020 | TF40024 | TF40028 | TF40032 | TF40036 | TF40040 | TF40043 | TF40047 | 5.5 |
| 4.5 | TF45008 | TF45013 | TF45016 | TF45020 | TF45024 | TF45028 | TF45032 | TF45036 | TF45040 | TF45043 | TF45047 | 5.5 |
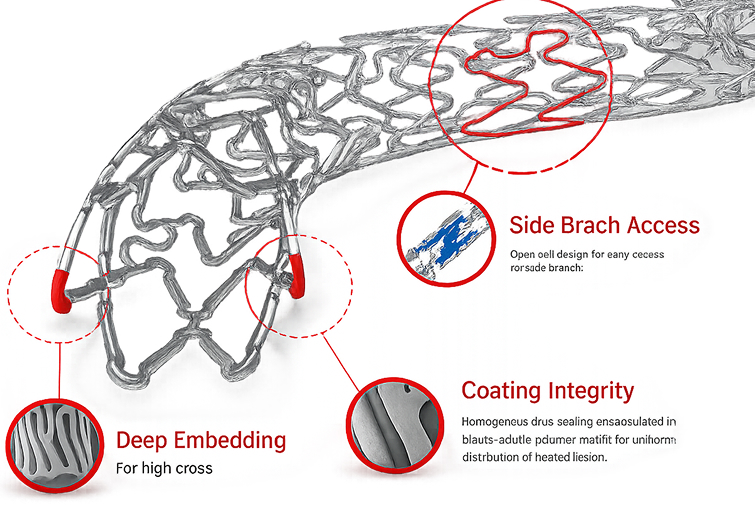
| Design | Open cell with unique alternate LDS link |
|---|---|
| Material | L-605 Cobalt Chromium |
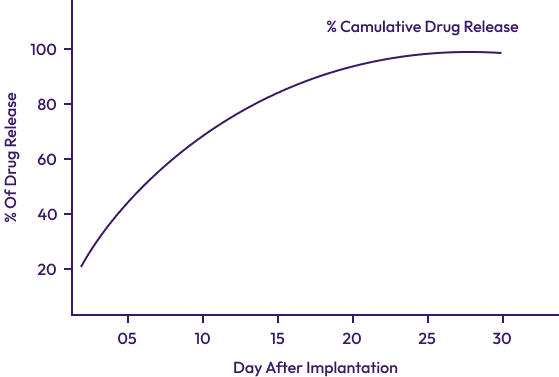
| Drug | Everolimus |
| Drug Dose | 1.2 µm m² |
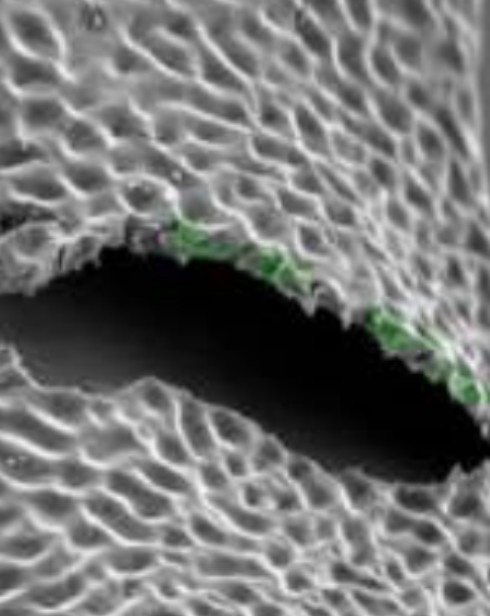
| Polymer | Biodegradable and Biocompatible |
| Strut Thickness | 65 µ |
| Diameter (mm) | 2, 2.25, 2.5, 2.75, 3, 3.5, 4 & 4.5 |
| Length (mm) | 8, 13, 16, 20, 24, 28, 32, 36, 40, 43 & 47 |
| Recoil | ≤ 5% |
| Delivery System | Rapid Exchange |
| Tip Entry Profile (mm) | 0.016” |
| Crossing Profile (mm) | 0.038” (3x20mm) |
|---|---|
| Guide Catheter Compatibility | 5 F |
| Guidewire Compatibility | 0.014” |
| Nominal Pressure | 9 atm |
| Rated Burst Pressure | 16 atm |
| Shaft Length | 145 cm |
| Balloon Overhang | ≤ 0.5 mm |
| Proximal Shaft Diameter† | 2.13 F |
| Distal Shaft Diameter† | 2.7 F |